Not actual size.

ABOUT DURYSTA®
First and only biodegradable, sustained-release,
intracameral implant1
A RELIABLE IOP-LOWERING INTERVENTION
FOR YOUR TOOLKIT

DURYSTA® is a straightforward therapy that is placed directly in the patient’s eye.1
Please see accompanying Prescribing Information for full administration steps.
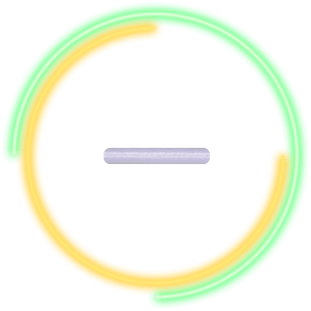
Implant1,2
- Solid polymer matrix containing 10 mcg of bimatoprost
- Slowly biodegrades in the eye
- Preservative-free and ~ 1 mm in length
- Designed to release bimatoprost for 3 to 4 months

Not actual size.
Implant1,2
- Solid polymer matrix containing 10 mcg of bimatoprost
- Slowly biodegrades in the eye
- Preservative-free and ~ 1 mm in length
- Designed to release bimatoprost for 3 to 4 months
Applicator1,3
- Sterile applicator designed for single use
- Preloaded with 1 DURYSTA® implant
- 28-gauge needle

Discover the pivotal
data behind this
therapy

Where DURYSTA®
may fit in the
treatment paradigm

Access DURYSTA®
resources for your
practice

Start the DURYSTA®
ordering process
today
IOP = intraocular pressure.
References: 1. DURYSTA® Prescribing Information. 2. Weinreb RN, Bacharach J, Brubaker JW, et al. Bimatoprost implant biodegradation in the phase 3, randomized, 20-month ARTEMIS studies. J Ocul Pharmacol Ther. 2023;39(1):55-62. 3. Medeiros FA, Walters TR, Kolko M, et al; ARTEMIS 1 Study Group. Phase 3, randomized, 20-month study of bimatoprost implant in open-angle glaucoma and ocular hypertension (ARTEMIS 1). Ophthalmology. 2020;127(12):1627-1641. doi:10.1016/j.ophtha.2020.06.018.


