CLINICAL DATA
DURYSTA® is a powerful IOP-lowering therapy
for patients with OAG or OHT.
In clinical studies, DURYSTA® demonstrated an IOP reduction of approximately 5 to 8 mm Hg in patients with a mean baseline IOP of 24.5 mm Hg.
Please see below for additional details.
EFFICACY DATA: ARTEMIS 1 AND 2 (PIVOTAL STUDIES)

Most DURYSTA® participants in the pivotal trials had mild OAG or OHT in early disease.1,2



Study Design1-3
Efficacy was evaluated in 2 multicenter, randomized, parallel-group, controlled, 20-month (including 8-month extended follow-up) studies of DURYSTA® compared to twice-daily topical timolol 0.5% drops in patients with OAG or OHT. IOP values obtained after initiation of nonstudy IOP-lowering treatment were excluded from the analysis.
Key exclusion criteria3,4
- History of narrow angle (Shaffer grade < 3)
- Nonresponsive to topical beta-blockers and/or PGAs therapy
- Peripheral anterior synechiae in the inferior iridocorneal angle on gonioscopic examination at screening in either eye
- History or evidence of complicated cataract surgery or phakic IOL insertion
- Contraindications to beta-blocker therapy
Key inclusion criteria3,4
- Patients ≥ 18 years of age with a diagnosis of OAG or OHT in each eye and both eyes requiring IOP-lowering treatment
- Study eye inferior iridocorneal angle Shaffer grade angle of ≥ 3 on gonioscopy and peripheral anterior chamber depth of ≥ 1/2 corneal thickness by Van Herick estimation
- Central corneal endothelial cell density by specular microscopy of ≥ 1800 cells/mm2 in at least 1 eye
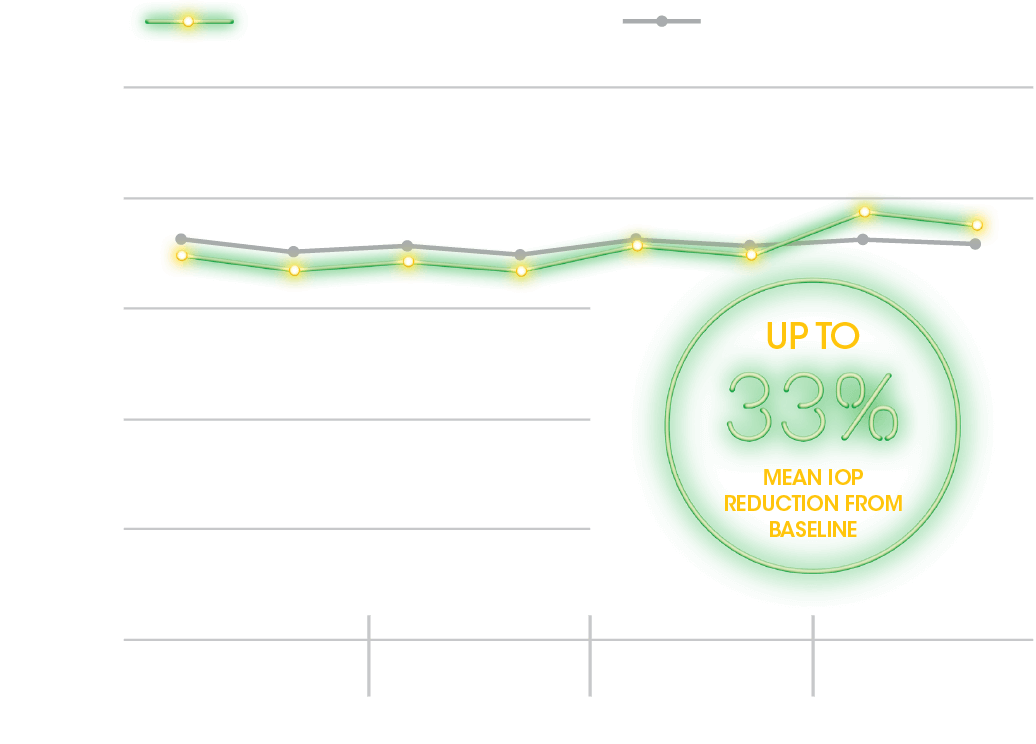
APPROXIMATELY 5 to 8 mm Hg mean IOP reduction over 15 weeks in
patients with a mean baseline IOP of 24.5 mm Hg1
Most common adverse reactions

Ocular
- The most common ocular adverse reaction was conjunctival hyperemia, which occurred in 27% of patients. Other common ocular adverse reactions reported in 5% to 10% of patients were foreign body sensation, eye pain, photophobia, conjunctival hemorrhage, dry eye, eye irritation, IOP increased, corneal endothelial cell loss, vision blurred, and iritis1


Nonocular
- The most common nonocular adverse event was headache, which was observed in 5% of patients1

ADDITIONAL DATA: MORPHEUS PHASE 3B SLEEP STUDY

Study Design5
The MORPHEUS study was a multicenter, open-label, noncomparative, phase 3B sleep lab study. Participants (N = 31) were administered a single DURYSTA® in study eye on Day 1. Study participants were nonsmoker adults with OAG or OHT, an open iridocorneal angle, and baseline IOP after washout of 22 to 34 mm Hg by GAT in at least 1 eye (the study eye). A Reichert Model 30 pneumatonometer was used when IOP measurements were collected at sleep lab visits and a GAT was used when IOP measurements were collected at office visits. While rescue IOP-lowering treatment could be initiated at the investigator’s discretion, IOP measurements were evaluated in study eyes that had not been rescued.
Primary endpoint5
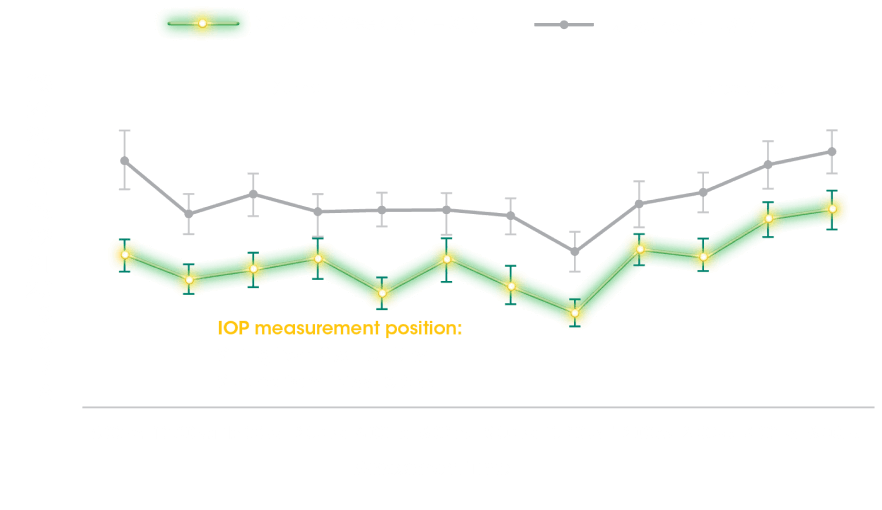
- Hour-matched change from baseline in habitual position IOP over 24 hours at Week 8 after being assessed with pneumatonometry
Secondary endpoint5
- Change from baseline in range of IOP in eyes treated with DURYSTA® at the Week 8 sleep lab visit
Other endpoint5
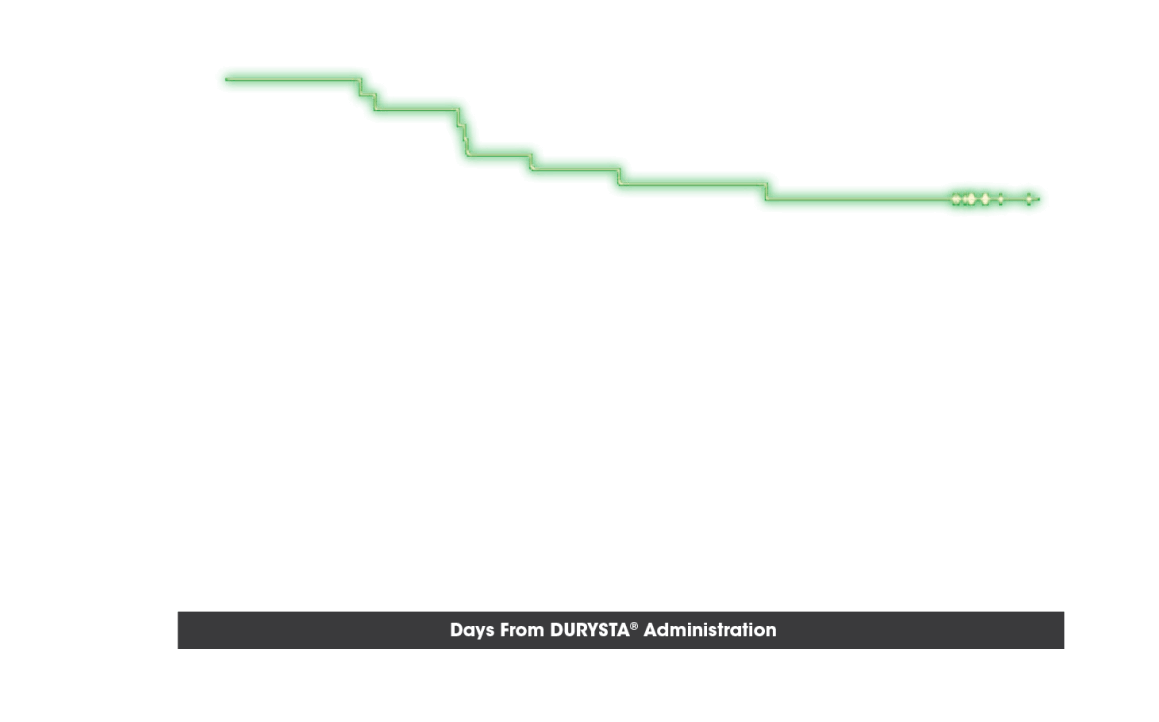
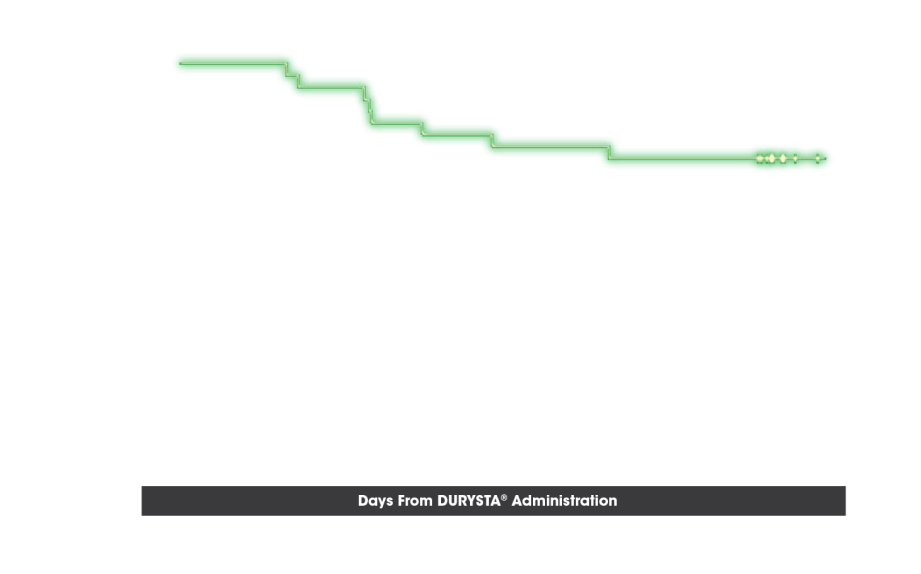
- Time to initial use of nonstudy IOP-lowering treatment
Study Limitations5
- Small number of subjects (N = 31) is not powered for statistical significance
- Nonrandomized, single-arm, unmasked study may introduce bias related to overall treatment effect. Interpret data with caution
Data limitations: There are no statistical hypotheses defined in the protocol for the study. Analysis results are based on descriptive statistics; therefore, no statistical conclusion can be drawn from this study.
PRIMARY ENDPOINT
24-Hour Habitual IOP Curve at Baseline and Week 85
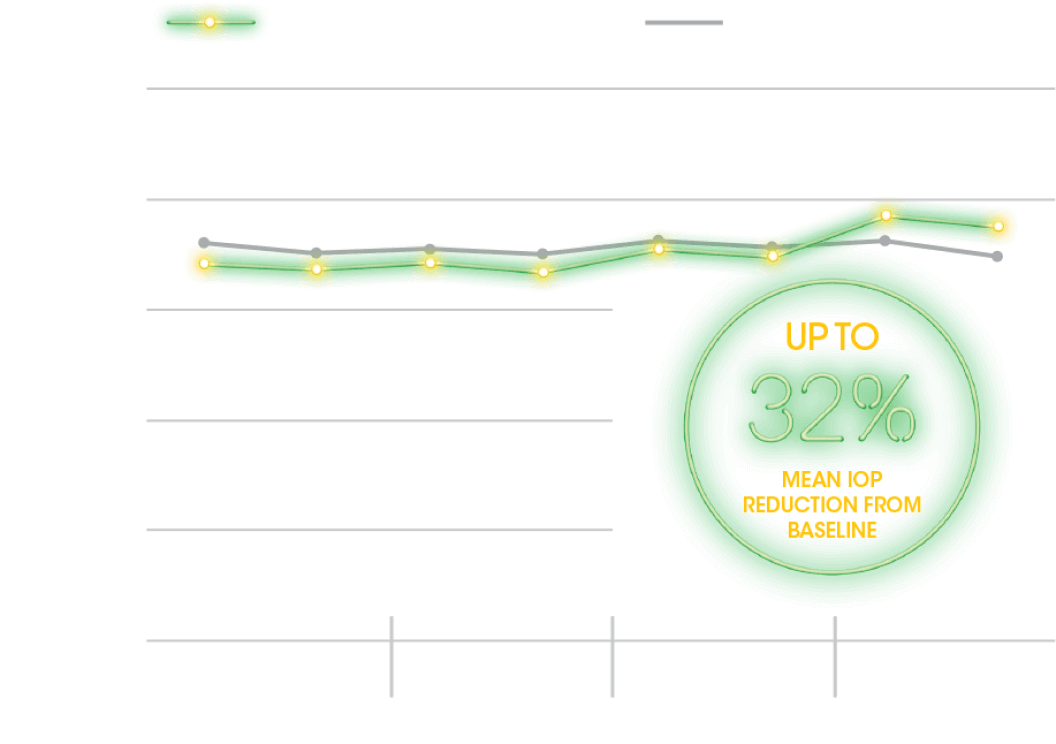
SECONDARY ENDPOINT
Range in Habitual IOP Over 24 Hours5
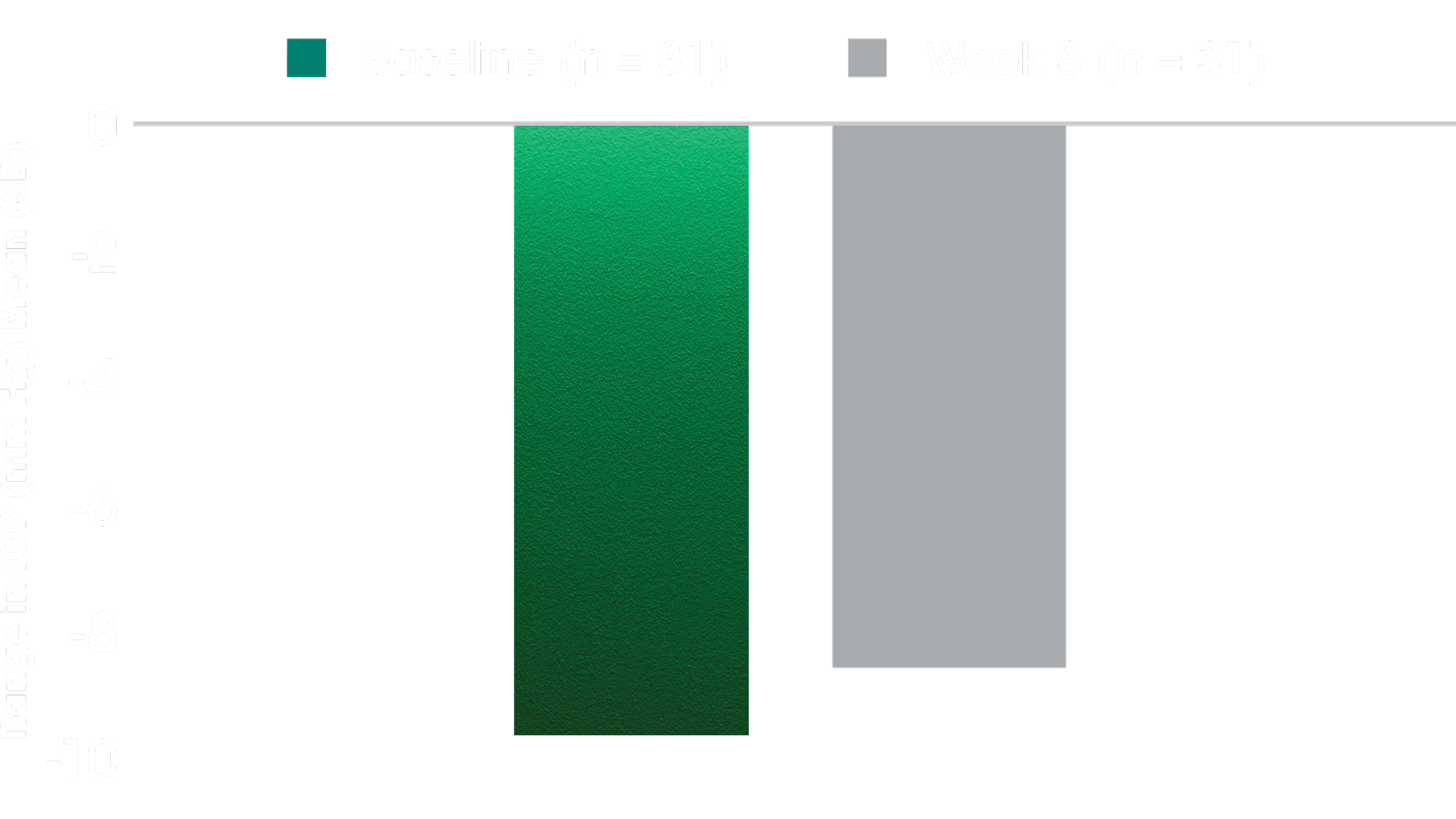
- The secondary outcome measure was change from baseline in range of IOP in eyes treated with DURYSTA® at the week 8 sleep lab visit5
- Habitual position IOP was measured using the range of habitual position IOP values for each participant collected from the sleep lab visit during the diurnal/wake period (8 am-10 pm), the nocturnal/sleep period (12 am-6 am), and the entire 24-hour period (8 am-6 am)5
Data limitations: There are no statistical hypotheses defined in the protocol for the study. Analysis results are based on descriptive statistics; therefore, no statistical conclusion can be drawn from this study.
OTHER ENDPOINT: TIME TO RESCUE
Probability of Not Requiring Rescue IOP-Lowering Treatment5
Additional Considerations
- The registrational trials that helped to support the FDA approval (ARTEMIS 1 and 2) demonstrated IOP reduction with DURYSTA® through 15 weeks.1 Additionally, the biodegradable implant releases medication for several months2; therefore, continued IOP monitoring is needed
Safety Data
- The most common TEAE was conjunctival hyperemia (incidence 35.5%, 11/31)5
- Intraocular pressure increased was reported in 22.6% (7/31) of cases5
- Other most common TEAEs reported in 6.5% to 9.7% of cases were conjunctival hemorrhage, visual field defect, cataract, dry eye, foreign body sensation in eye, punctate keratitis, and COVID-195

Learn how DURYSTA®
works for several
months

Where DURYSTA®
may fit in the
treatment paradigm


Start the DURYSTA®
ordering process
today
BID = twice per day; OAG = open-angle glaucoma; OHT = ocular hypertension; PGA = prostaglandin analog; GAT = Goldmann application tonometer; TEAE = treatment-emergent adverse events.
References: 1. DURYSTA® Prescribing Information. 2. Medeiros FA, Sheybani A, Shah MM, et al. Single administration of intracameral bimatoprost implant 10 μg in patients with open-angle glaucoma or ocular hypertension. Ophthalmol Ther. 2022;11(4):1517-1537. doi:10.1007/s40123-022-00527. 3. Medeiros FA, Walters TR, Kolko M, et al; ARTEMIS 1 Study Group. Phase 3, randomized, 20-month study of bimatoprost implant in open-angle glaucoma and ocular hypertension (ARTEMIS 1). Ophthalmology. 2020;127(12):1627-1641. doi:10.1016/j.ophtha.2020.06.018. 4. Bacharach J, Tatham A, Ferguson G, et al; ARTEMIS 2 Study Group. Phase 3, randomized, 20-month study of the efficacy and safety of bimatoprost implant in patients with open-angle glaucoma and ocular hypertension (ARTEMIS 2). Drugs. 2021;81(17):2017-2033. doi:10.1007/s40265-021-01624-9. 5. Weinreb RN, Christie WC, Mederios FA, et al. Single administration of bimatoprost implant: effects on 24-hour intraocular pressure and 1-year outcomes. Ophthalmol Glaucoma. 2023;6(6):599-608. doi:10.1016/j.ogla.2023.06.007.